Baking soda is a typical component of baked goods, but have you ever stopped to consider whether it behaves more like an acid or a base? In this piece, we will go into the chemistry underlying baking soda and its qualities, as well as the history of the substance. & explain ‘Is baking soda acid or base?’
What is Baking Soda?
The chemical term for baking soda is sodium bicarbonate, which appears as a white crystalline powder (NaHCO3). Leavening agents are utilised rather often in the production of baked products such as cakes, cookies, and biscuits. Baking soda achieves its effects in baked goods by a chemical reaction with acids present in the batter. This reaction results in the production of carbon dioxide gas, which causes the baked products to rise and become airy.
Na2CO3 +CO2 +H2O→2NaHCO3
2 NaHCO3 → Na2CO3 + H2O + CO2
What is the purpose of baking soda?
Baking soda can do what it’s supposed to do when it’s mixed with something acidic, like vinegar, lemon juice, or buttermilk. Baking soda and acid have a chemical reaction that results in the production of carbon dioxide gas, which causes the batter to rise when it is baked. The gas bubbles cause the batter to expand, resulting in a more airy and light texture. Let’s find the answer Is Baking Soda Acid or Base?
Is baking soda acid or base?
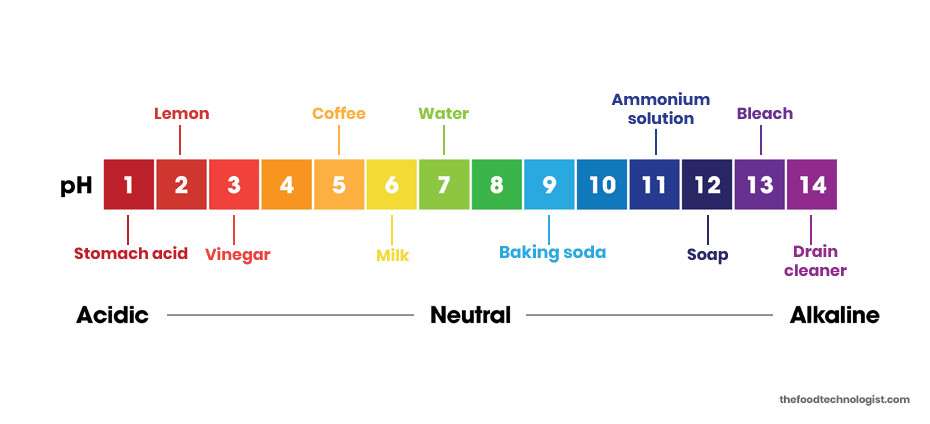
Is Baking Soda Acid or Base? Baking soda has a pH ranging from 8.5 to 9, making it a base. Because it is naturally alkaline, the pH of the solution is basic. When one per cent molar of baking soda is dissolved in water, the pH ranges from 8.4 to 9, which means that the solution is alkaline. Baking soda, although an alkaline substance, has a taste that is slightly acidic and salty.
Baking Soda vs. Baking Powder
Baking powder and baking soda are frequently confused with one another, despite the fact that they are not the same item. Baking soda, cream of tartar (a dry acid), and occasionally cornflour are the three components that makeup baking powder. Cream of tartar is one of the ingredients in baking powder. It acts as an acid when baking soda and baking soda powder react, which makes carbon dioxide gas. Baking powder already has an acid in it, so it may be used in recipes that don’t ask for an acidic ingredient. This is because baking powder includes an acid that can negate the effects of baking soda.
Additional Applications of Sodium Bicarbonate
Baking soda has a wide variety of applications outside of the realm of baking. When combined with water, it functions both as an all-natural deodorizer and as a gentle cleaning solution that won’t scratch surfaces. Baking soda has several uses, including reducing the discomfort of heartburn and making the air smell better. Sodium bicarbonate is a light abrasive cleanser that may be used on carpets, countertops, and sinks to get rid of stains and smells. It is also a mild abrasive, thus it is used in toothpaste to get rid of plaque.
In conclusion, baking soda is a fundamental substance that also has an alkaline component. It accomplishes this by undergoing a chemical reaction with the acids present in the batter, which results in the production of carbon dioxide gas. This gas causes the baked items to rise and become airier. Baking powder is not the same thing as baking soda, yet baking soda is one of the ingredients in baking powder. Baking powder is used to make carbon dioxide gas by combining baking soda with an acid. Baking soda is a multipurpose substance, making it an invaluable addition to the pantry of any home outside the kitchen.




Very helpful