The Food Safety and Standards Authority of India (FSSAI) has issued the Food Safety and Standards (Labelling and Display) Regulations, 2020, in a notification dated November 18, 2020. (Final Regulations). Last year, the draught regulations in this area were published. The Final Regulations have now been issued after taking into account the comments submitted.
Food Business Operators (FBOs) must comply with all provisions of the FSSAI Labelling and Display Regulations after one year from the date of their publication in the Official Gazette, i.e. 17.11.2021. The Regulations suppress the Food Safety and Standards (Packaging and Labelling) Regulations, 2011 and are effective immediately. On the other hand, FSSAI can complete chapter 3 on the information display in foodservice outlets by January 1, 2022.
Key Highlights under the Final FSSAI Labelling and Display Regulations:
- When a food product is sold through e-commerce or any other direct selling method, the obligatory label requirements must be disclosed to the consumer through proper ways prior to sale, according to the regulations. The following items, however, are exempted from this requirement under the Final Regulations: batch number/lot number, best before, use by date, expiry date, and date of manufacturing/packaging.
- When a compound ingredient is made up of two or more components, it must be reported by their
- Unique names in the ingredient list, or
- By reporting all of the compound ingredient’s ingredients as if they were individual ingredients in the finished item.
- When a compound component makes up less than 5% of the food, it is not required to be declared, with the exception of food additives that perform a technological role in food items.
- Only if the fat level is more than 0.5 percent must nutritional information be provided in the case of saturated and trans fat.
- Foods that claim to be enriched with nutrients like minerals, proteins, vitamins, amino acids, or enzymes must list the amounts of those added nutrients on the label.
- The list of items that are excluded from the statutory nutritional labelling requirement has been completely updated.
- The amount of energy that must be listed must be determined using specific conversion factors. Dietary fibre 2kcal/g is a novel conversion factor in this regard.
The Requirements of display of FSSAI license are specified.
Moreover, The brand Owner must place the FSSAI logo and the brand owner’s licence number on the label. In addition, if the manufacturer, marketer, packer, or bottler is not the same as the brand owner, Brand Owner must display the licence number of the manufacturer, marketer, packer, or bottler on the label.
Also, The FSSAI emblem and licence number and the importer’s name and address must be displayed on imported food goods.
FBOs must post the Registration/License No. or Food Safety Display Board if one is required and any other information required by the FSSAI in a prominent location on all of their facilities where food is stored, processed, distributed, or sold.
The logo prescribed in Schedule II of the Regulations must be applied to fortified and organic foods. FSSAI may, from time to time, specify a logo for any other food.
Display of ‘Nutritional Information’ -FSSAI Labelling and Display Regulations
For these regulations, nutritional information is a description intended to inform the consumer of the nutritional properties of the food, and the following definitions are applicable:
(i) ‘sugars’ means all monosaccharides (glucose, fructose, etc.) and disaccharides (maltose, sucrose, lactose, etc.).
(ii) ‘added sugars ‘means monosaccharides and disaccharides added to foods and beverages
(iii) ‘fat’ means total lipids, including saturated fat, monounsaturated fat, polyunsaturated fat and trans fat.
- ‘Saturated fats’ means fatty acids without double bonds.
- ‘Monounsaturated fats’ means fatty acids with one cis double bond.
- ‘Polyunsaturated fats’ means fatty acids with cis-cis methylene interrupted double bonds.
- ‘Trans fat’ means all the geometrical isomers of monounsaturated and polyunsaturated fatty acids having non-conjugated, interrupted by at least one methylene group, carbon-carbon double bonds in the trans configuration.
(iv) “dietary fibre” means carbohydrate polymers with a degree of polymerization (DP) not lower than three, which are not hydrolysed by the endogenous enzymes in the small intestine of humans and the same consists of one or more of-
- Edible carbohydrate polymers naturally occurring in the food as consumed;
- Carbohydrate polymers, which have been obtained from food raw material by physical, enzymatic or chemical means;
- Synthetic carbohydrate polymers.
(v) ‘nutrient’ means a constituent of food, which:
- provides energy; or
- has specific metabolic or physiological functions; or
- is needed for growth and development and maintenance of healthy life;
(b) Nutritional Information per 100g or 100ml or per single consumption pack of the product and per serve percentage (%) contribution to Recommended Dietary Allowance calculated based on 2000kcal energy, 67 g total fat, 22 g saturated fat, 2 g trans fat, 50 g added sugar and 2000 mg of sodium (5 g salt) requirement for an average adult per day, shall be given on the label containing the following: —
- energy value (kcal);
- the amounts of:
- Protein (g);
- Carbohydrate (g) and Total Sugars (g), added sugars (g);
- Total fat (g), saturated fat (g), trans fat (other than naturally occurring trans fat) (g) and cholesterol (mg);
Provided that FBOs may declare saturated fat and trans fat content on the label as “not more than”.
Provided that saturated fat and trans fat to be given only if the fat content is more than 0.5%
4. Sodium (mg);
(iii) Wherever numerical information on vitamins and minerals is declared, it shall be expressed in metric units;
(iv) Nutrition information panel shall include the amount of food in gram (g) or millilitre (ml) for reference beside the serving measure and the number of servings in the package.
Explanation: “serving or serve size” means an amount of food customarily consumed per eating occasion or defined on the label, expressed in the metric unit. Additionally, it may also be given in common household measures like a teaspoon, tablespoon, and the appropriate cup for the food.
Provided that the food claimed to be enriched with nutrients, such as minerals, proteins, vitamins, amino acids, or enzymes, shall give the quantities of such added nutrients on the label.
Food Exempted from Mandatory Nutritional Labelling:
The following foods are exempted from mandatory nutritional labelling:
- Unprocessed products that comprise a single ingredient;
- Processed products which the only processing they have been subjected to is maturing and that comprise a single ingredient;
- Waters intended for human consumption, including those where the only added ingredients are carbon dioxide;
- An herb, a spice or mixtures thereof/Curry Powder except Sprinkler masala (masalas meant for direct consumption);
- Salt and salt substitutes;
- Table top sweeteners;
- Coffee extracts and chicory extracts, whole or milled coffee beans and whole or milled decaffeinated coffee beans, coffee, decaffeinated coffee, soluble coffee powder, coffee chicory mixture;
- Herbal and fruit infusions, tea, decaffeinated tea, instant or soluble tea or tea extract, decaffeinated instant or soluble tea or tea extract, which do not contain other added ingredients than flavourings which do not modify the nutritional value of the tea;
- Fermented vinegars and substitutes for vinegar, including those where the only added ingredients are flavourings;
- Flavourings, Food additives, Processing aids, Food enzymes, Gelatine, Yeast;
- Chewing-gums;
- Alcoholic Beverages.
- Foods for Special Dietary Uses (FSDU), Foods for Special Medical Purposes (FSMP), subject to the compliance of requirements specified in the Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purpose, Functional Food and Novel Food) Regulations, 2016.
Provided that every package of edible oils, interesterified vegetable fat, both hydrogenated or partially hydrogenated oils, edible fats, margarine and fat spreads (mixed fat spread and vegetable fat spread) and a package of food in which fats, oils and fat emulsions are used as an ingredient shall declare the quantity of trans fat content and saturated fat content on the label.
Saturated fat and trans fat may be declared on the label as “not more than”.
Provided further that every package of edible oils, interesterified vegetable fat, both hydrogenated or partially hydrogenated oils, edible fats, margarine and fat spreads (mixed fat spread and vegetable fat spread) shall declare the quantity of monounsaturated fatty, polyunsaturated fatty acid, omega-3 fatty acid and omega-6 fatty acid content on the label.
However, nutritional information shall be required in the above-mentioned products if a nutrition or health claim is made on the label.
(d) The compliance to the number of declared nutrients on the label shall have the tolerance of a maximum minus 10 per cent of the value for that nutrient declared on the label at any point in time within the declared shelf life of the product.
Calculation of Nutrients:
(i) Calculation of Energy: The amount of energy to be listed should be calculated by using the following conversion factors:
(A) Carbohydrates 4 kcal/g
(B) Polyols except Erythritol 2 kcal/g
(C) Erythritol 0 kcal/g
(D) Protein 4 kcal/g
(E) Fat 9 kcal/g
(F) Alcohol (Ethanol) 7 kcal/g
(G) Organic acid 3 kcal/g
(H)Dietary fibre 2 kcal/g
(ii) Calculation of Protein – The amount of protein to be listed should be calculated using the formula:
Protein = Total Kjeldahl Nitrogen x 6.25 (Unless a different factor scientifically justified, may be used)
Provided that for calculating protein content in milk, a conversion factor of 6.38 needs to be used.
(f) Food labels may additionally provide nutritional information in the form of a Barcode/Global Trade Identification Number (GTIN).
More Focus on ‘PDP’ (Principle Display Panel)- FSSAI Labelling and Display Regulations
The symbols for vegetarian and non-vegetarian foods have been updated:
Principal display panel means that part of the container/package which is intended or likely to be displayed or presented or shown or examined by the customer under customary conditions of display, sale or purchase of the food article contained therein
According to the Draft Regulation, the symbol for vegetarian foods containing chemicals such as food additives and plant processing aids was a green triangle inside a square. It’s now a green-filled circle inside a square.
According to the Draft Regulation, a brown circle inside a square denoted non-vegetarian food, including components such as food additives. It’s now a brown-coloured triangle within a square.

A new declaration requirement has been added. Every package of foodstuff that is not intended for human consumption must now have a declaration to that effect, represented by a black cross inside a square with a black outline.

In terms of declaring one’s name and address, it’s been emphasised that alcoholic beverages must be labelled with the words “Bottled by” or “Blended and Bottled by” or “Imported and Bottled by.” On the label, it might say “distilled and bottled by.”
In Addition, There are provisions for making allergy statements on foods and ingredients that are known to cause allergies.
Declaration regarding Food allergen:
The following foods and ingredients which are known to cause allergy shall be declared separately as Contains………………………. (Name of allergy-causing ingredients)
- (i) Cereals containing gluten; i.e., wheat, rye, barley, oats, spelt or their hybridized strains and products of these (To be declared as the name of the cereal);
- (ii) Crustacean and their products (To be declared as Crustacean);
- (iii) Milk & Milk products(To be declared as Milk);
- (iv) Eggs and egg products (To be declared as Egg);
- (v) Fish and fish products (To be declared as Fish);
- (vi) Peanuts, tree nuts (e.g. almonds, walnuts, pistachio, cashew nuts) and their products (To be declared as Nut);
- Soybeans and their products (To be declared as Soy);
- Sulphite in concentrations of 10mg/kg or more (To be declared as sulphite)
Provided that in case presence of ingredients due to cross-contamination which is known to cause
allergy may be declared separately as May Contains…………………….. (Name of allergy-causing ingredients).
Provided further that this declaration is not required in case of oils derived from these ingredients.”
Raw agricultural commodities are exempted from the allergen labelling requirements.
It is stated that, in the case of assorted packs, the shelf life indicated on the assorted pack must be that of the product with the earliest declared shelf life among the several pre-packaged foods contained therein.
Food Service Establishments must include the following information next to the food products on menu cards or boards (the criteria are less stringent than in the Draft Regulations): As prescribed, information about food allergies. Simple symbols can also represent allergens. vegetarian or non-vegetarian logo
Every package of fortified food shall carry the words “fortified with ………… (name of the fortificant)” and the logo, as specified below, on the label. It may also carry a tag line “Sampoorna Poshan Swasth Jeevan” under the logo.
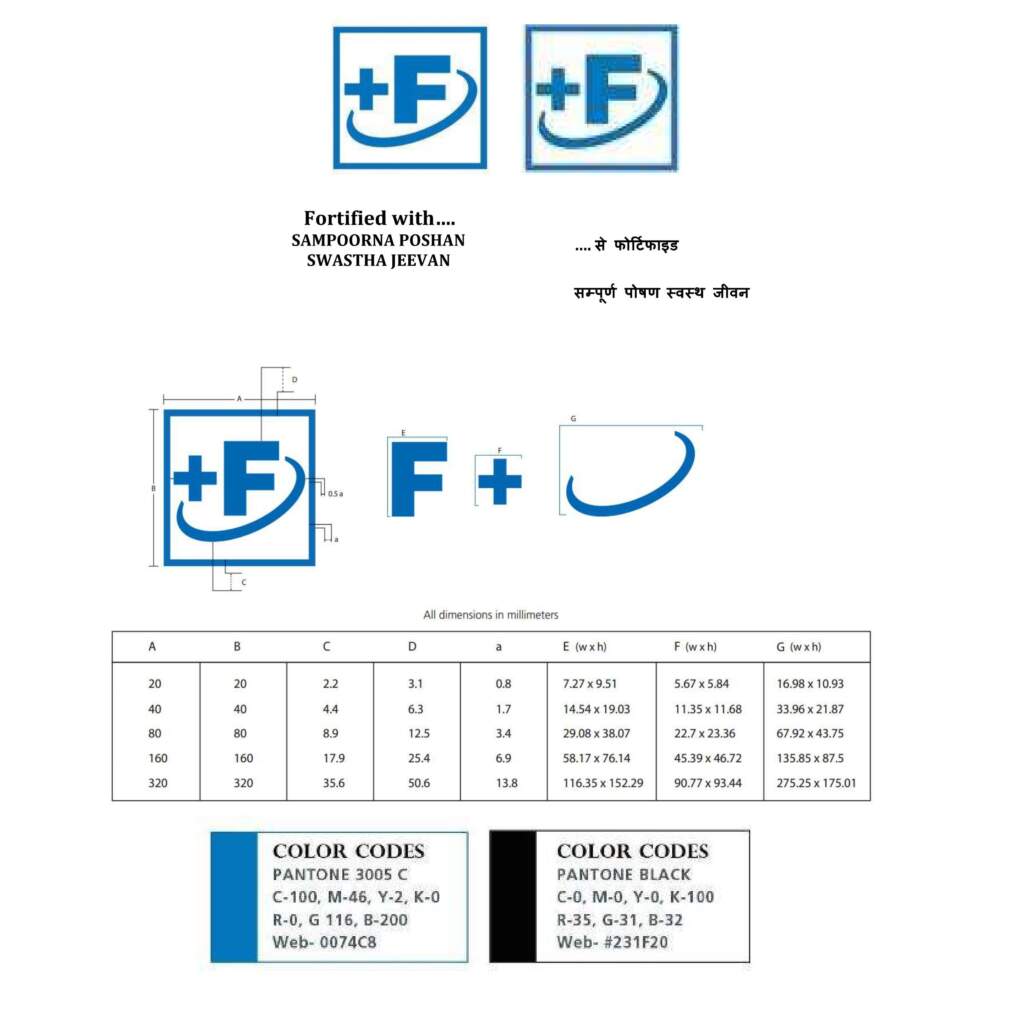
and the logo. (Source: FSSAI)
Every package of certified organic food as per Food Safety and Standards (Organic Foods) Regulations, 2017 shall carry the logo as specified below:
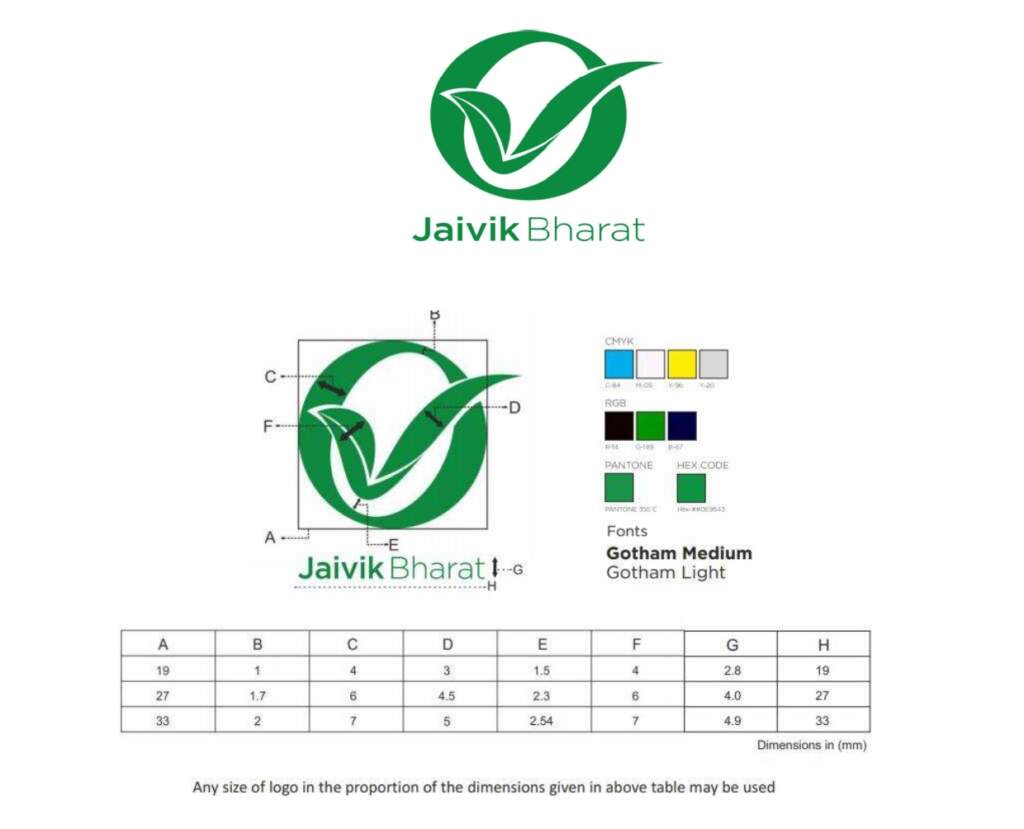
2017 shall carry the logo.
References:
Food Safety and Standard Authority of India (FSSAI). Food Safety and Standards (Labelling and Display) Regulations, 2020. Read the full Gazzete notification here.












Leave a Comment